-
When calling `QL:UPDATE-ALL-DISTS` and `QL:SYSTEM-APROPOS` I'm getting the "No applicable methods" error, `CLOS:SLOT-BOUNDP-USING-CLASS`, in LispWorks Enterprise v7.1.2, 64-bit, on Windows 10, but not…
-
This is a major functionality which in my mind is missing from OpenBabel. The code is already included in [mychem](https://github.com/mychem/mychem-code/blob/master/src/serialization.cpp) by @Pansanel…
-
**Steps to Reproduce**
1. Draw a 5-membered, nitrogen-containing aromatic ring (e.g. pyrrole, indole, etc.).
2. Click on the aromatization button
3. Look at the represented molecule structure and e…
-
Can you provide a full environment setup please ?
I notice several errors from rdkit that break the code, this is during prepare_data step using ubuntu linux:
I fix few issue to make it run un…
-
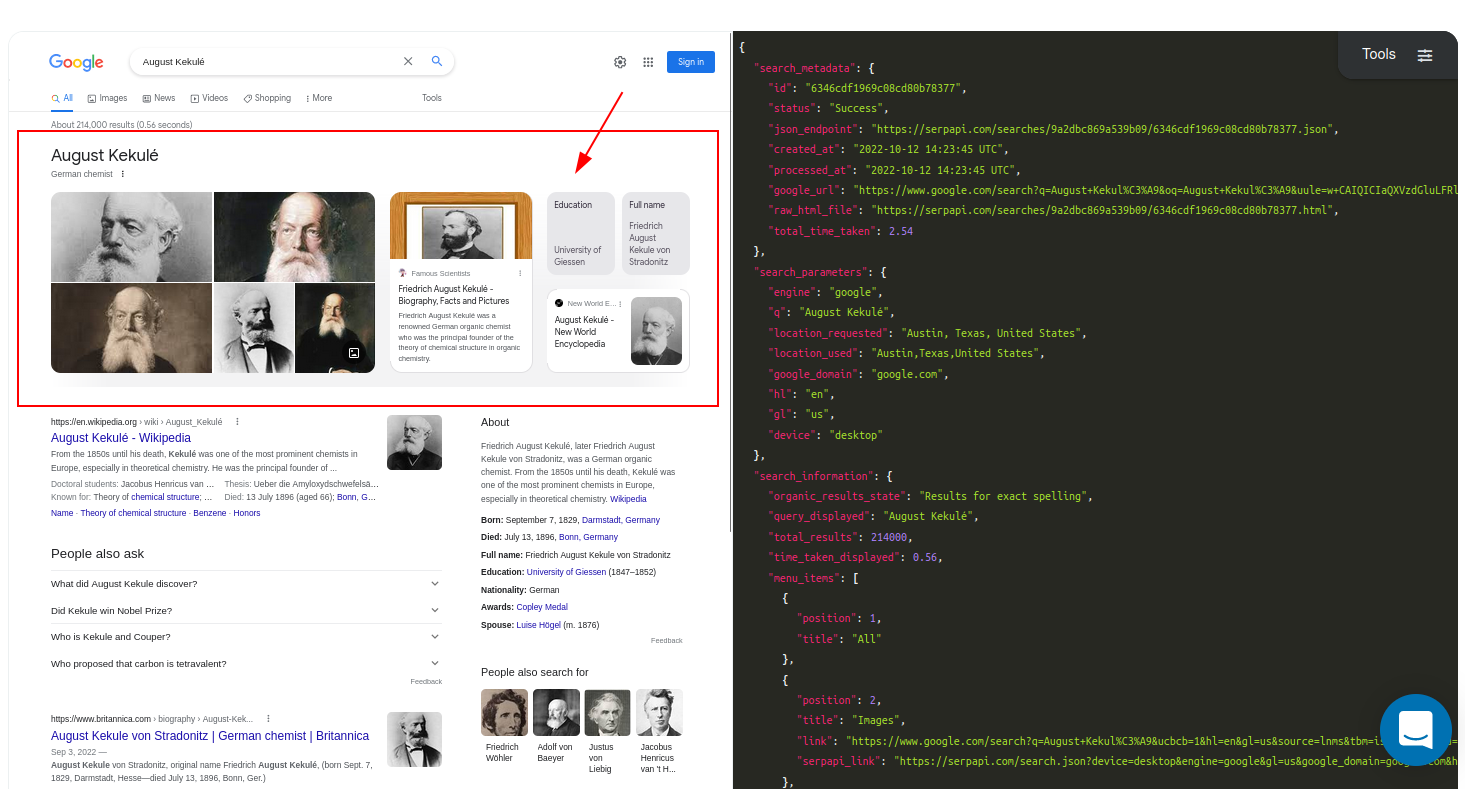
[Playground](https://serpapi.com/playground?q=August+Kekul%C3%A9&location=Austin%2…
-
The original self claimed intent of the OXC-LPCorrectr was to be open hardware.
Per the definition of open source the EDA software design files should be supplied. (E.g KiCad .kicad_pcb .kicad_sch a…
-
During SMARTS creation, the C termini seem to have a bond perception problem, yielding things like
`[H][C@:3]1([C:2]([C:1](C(N1)([H])[H])([H])[H])([H])[H])[C-]([O-])[O-]`
This Issue is to figure …
-
**Describe the bug**
When drawing query molecules constructed from SMARTS that have partial, unclosed aromatic rings, some aromatic bonds are drawn as a dashed line overlaying a solid line instead of…
-
Dear developers,
I'm trying to run the example: cdw_1d
I can not run the python code cdw_1d .py because the wigner.fmt file is missing in my directory. Is that a file that epw is supposed to gi…
-
I have noticed that if the Composer widget is on the same webpage as a form element like an input or textarea, the composer event handlers appear to prevent backspace, delete, control-C, etc. from wor…