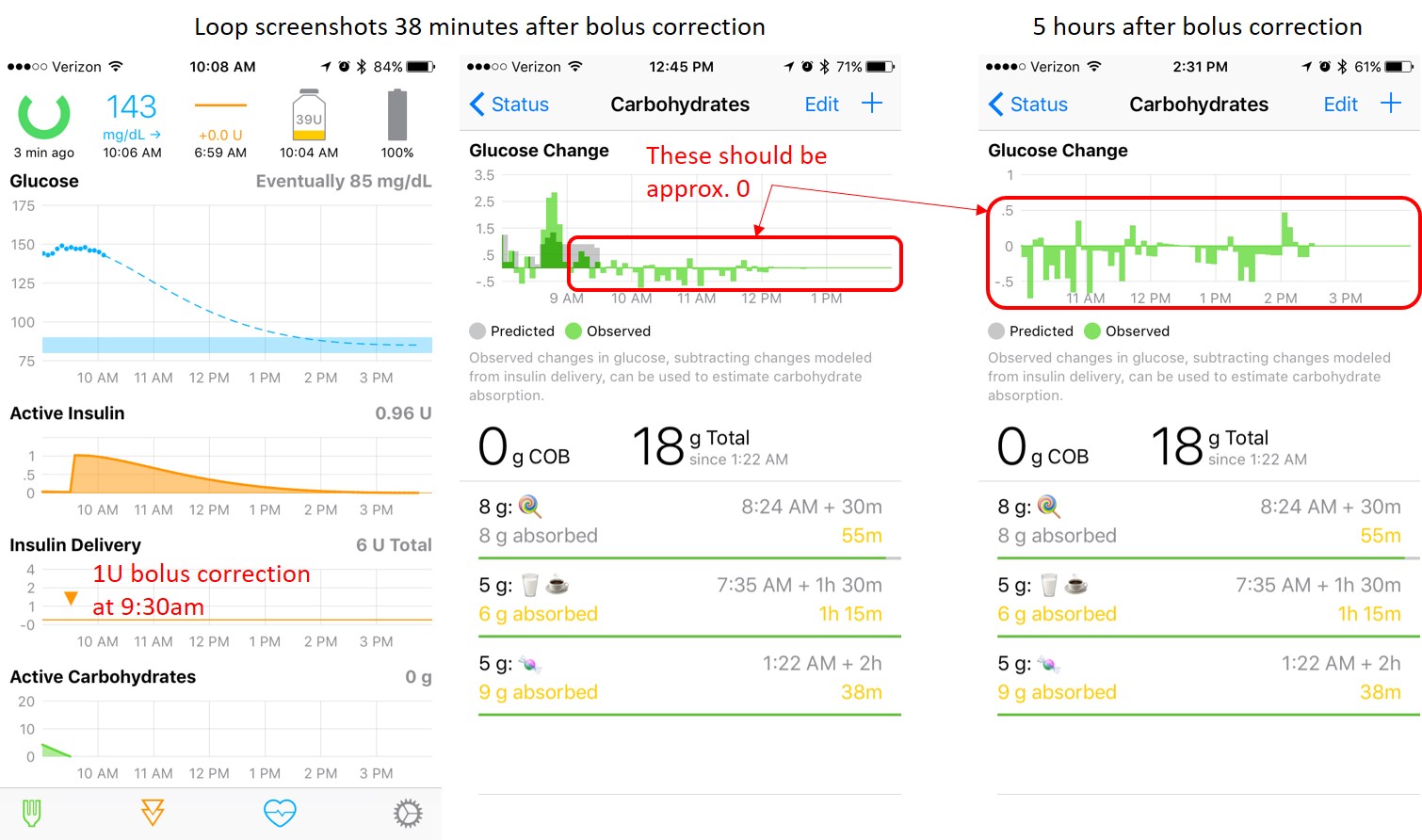
@dm61 <3 the constant AUC of the latest model. td=360 seems very long though? What was the td for the first pass model with the good curve fit, it's not mentioned in the post with the first curve fit images? When comparing the first model with the latest, with peak set to 55, the new new peaks at a much higher activity level than the previous model and the peak at 75 minutes, the new model peaks at a lower value, which makes me wonder which one is more correct. I'd assume people would like to set the td to somewhere between 4 to 5 hours?











Add setting to select type of insulin A) Novo/Humalog B) FIAsp
If A selected use current curve calculation If B selected use maths to match curve as described on page 12 of http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004046/WC500220890.pdf