Hi Violeta, A few comments first. The bioavailability of cipro from the popPK model is about 50% in adults (which is lower than the expected of 60-70%) and 94% in children. At least that is what I am getting from your popPK estimates. This is a high F in kids....from a previously published popPK model in a rather large age range of kids, F in kids (61%) is similar to adults (Rajagopalan. Population pharmacokinetics of ciprofloxacin in pediatric patients. J Clin Pharmacol). Using CL as the reference in children, the bioavailability as predicted from PK-Sim is 65%. If the model is built on adults (F = 61% in the adult model as reported from your numbers) and the drug is about 35% hepatically cleared (low extraction ratio of about 0.2) in adults (biliary is fully mature and 1A2 is not), 65% in children is a reasonable prediction. Considering that cipro is 2/3rds renally cleared in adults (more in kids), it would be suspect to predict 94% in kids.
The Vss/F is wonky. I recreated a cipro model (in general) and I can't recreate such as huge Vss difference between IV and oral. On a side note, the V is set in PK-Sim mechanistically meaning that there is one V that is derived for each compound, regardless of the administration. The V that you are looking at in the PK-Analysis window is derived as one would derive it from a concentration vs. time curve. Thus the V from the time curve is dependent on the how long the simulation goes for. Always make sure that you you are finishing the simulation at the same time as the observed data! For cipro, this makes a big difference because oral uptake occurs along the length of the intestines and, as such, 12 hours vs. 72 hours changes the dose arriving systemically (changing all PK parameters).
I have a few questions before I can figure out what's going on:-)
- How old are the kids in the trial? By the numbers I'm guessing in the 2-6 yr range?
- Did you create one virtual child for every real child and predict the profile and compare to observed levels? I think so, just checking.
- How did you incorporate the GFR for each kid? If you could give me an example, that would be helpful. As a check, you could just leave the GFR as estimated for the age of the child and see if the under-prediction in concentrations remains.
- How did you incorporate tubular secretion ontogeny?
Take care! Andrea
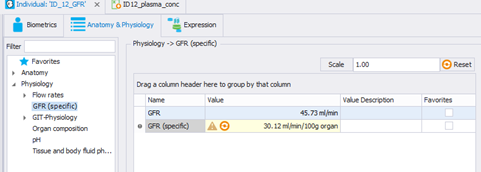 (The GFR has been calculated using the Schwartz formula non normalized by BSA, as we have the serum creatinine:
(The GFR has been calculated using the Schwartz formula non normalized by BSA, as we have the serum creatinine:

Dear all,
I would like to share some issues I have with my project and maybe anyone could have some suggestions about how can I improve my PBPK model.
I am trying to extrapolate to pediatric population an already existing model for adults in PKSim (Kuepfer L, Niederalt C, Wendl T, Schlender JF, Willmann S, Lippert J, et al. Applied Concepts in PBPK Modeling: How to Build a PBPK/PD Model. CPT Pharmacometrics Syst Pharmacol. 2016;5(10):516–31).
The drug (Ciprofloxacin), present its CL splitted in three different processes: Enzymatic: CYP1A2, Bile, and Renal (GFR + TS). I would like to know if there is any way in which I can know the CL prediction per CL pathway.
The adult model predicts very well plasma concentration for IV and PO administration. Also CL and Vss for IV administration are well predicted as well, comparing them with the PK parameters for adult population obtained from the different literature trials. But for PO administration, Vss/F is overpredicted.
ADULT IV
PO
Vss/F PBPK model= 855.66 L
Vss/F PK trials (range)= 332.36 (237-493.3)L REFERENCE
CL/F PBPK model=48.60 L/h
CL/F PK trials (range)=64.64 (51.9-70.6) L/h REFERENCE
Pred vs observed for the adult population (dashed line refers to the twofold time prediction interval):
When I extrapolate the model to pediatric population (in-house data with customized fu and GFR per ID), the model overpredicts Vss in IV and much more in PO (Vss/F) parameters, so we should figure out why (In that case, the reference parameters come from a popPK model that is being developed).
CHILDREN IV
PO
Pred vs observed for the adult population (dashed line refers to the twofold time prediction interval):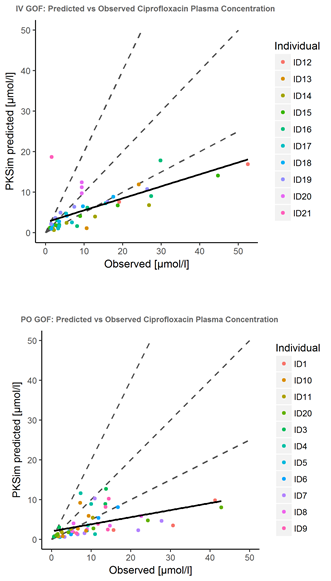
It seems that there is a problem overpredicting Vss, specially after PO administration. Any suggestions?
I also would like to know if there is any trick to obtain as an output of the simulation the CL prediction per CL pathway.
Thank you in advance Violeta