Originally posted by **pedrobtz** January 10, 2023
I would suggest to use a Design Thinking approach to this topic. I have the impression that we are already between "ideate" and "prototype" phase, and I would be interested in understanding better the "emphasize" and "idealize".
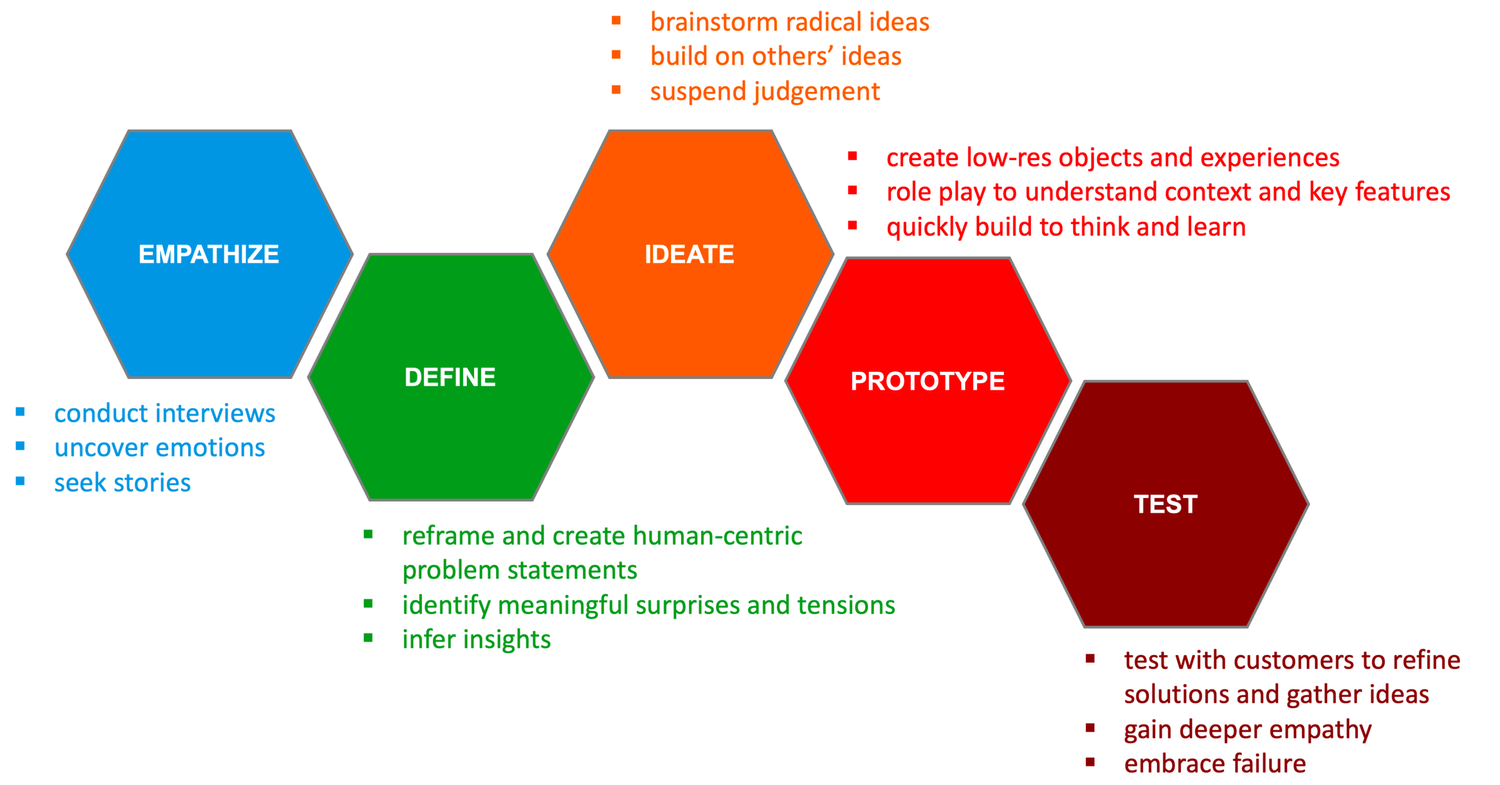
#### On Emphasize:
- One can collect & document Developers and Regulators feedback, ideas, etc.
#### On Idealize:
I see (at least) two user stories.
1. As a R developer of mathematical models [or statistical analsysis], for a regulated industry (Pharma, Banking, Insurance, etc.), I would like to be able to better evaluate R package quality.
2. As a company operating in a regulated environment, I would like to setup centrally an (internal or cross-industry) CRAN-like repository such that all R packages [which are used for regulated modelling activities] were (and are regularly) evaluated and guarantee to fulfil pre established quality requirements.
### References:
- https://www.designreview.byu.edu/collections/design-thinking-part-1-basic-concepts-and-principles
- https://web.stanford.edu/~mshanks/MichaelShanks/files/509554.pdf
Discussed in https://github.com/pharmaR/regulatory-r-repo-wg/discussions/31
Migrated following decision in #20